Abstract
Von Willebrand factor (VWF) is the largest protein in plasma and promotes the early hemostatic response following vascular injury. The hemostatic response of VWF relies on the exposure of its A1 domain, which recognizes its receptor GPIbα on the platelet surface. To avoid binding and activating platelets in the absence of injury, the A1 domain is dormant in full-length VWF through a mechanism that is not completely understood. We recently characterized two recombinant A1 fragments (longA1, residues 1238-1472; and shortA1, residues 1261-1472) with differential GPIbα-binding affinities by hydrogen-deuterium exchange (HDX) mass spectrometry. We found that N- and C-terminal residues flanking the A1 domain (residues 1238-1271 and 1459-1472) cooperatively constitute an autoinhibitory module (AIM) that masks a region in A1 and impedes its binding to GPIbα[1]. To determine if the A1 domain in full-length VWF is "masked" in the same manner, we have characterized human plasma-derived VWF using the same HDX approach.
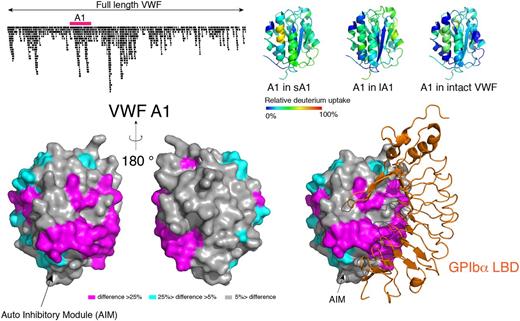
On-column peptic fragmentation was performed following HDX from 10 to 10,000 sec. We obtained 946 sequenced peptides covering 96.4% of the VWF sequence with an average redundancy of 6 per residue. Among them, 113 peptides covered the A1 domain, allowing HDX analysis of A1 at high resolution. We found that residues around the 1272-1458 disulfide bond, helix α1, loops α1β2 and β3α2 in the A1 domain, which are protected by the AIM in the longA1 fragment (residues 1238-1472), are deeply protected in full-length VWF, suggesting that AIM in full-length VWF likely masks A1 in a similar pattern. Additionally, sheet β3, helix α3, and loops β4α3 in the A1 domain of full-length VWF reported lower HDX rates than their counterparts in the shortA1 fragment. These regions, along with those protected by AIM, form a contiguous surface that significantly overlaps with the GPIbα-binding interface (Fig. 1). Residues in loop β6α6, helix α6 and helix α1 also reported lower HDX rates, but they are separate from the regions protected by AIM and the GPIbα-binding interface.
In summary, our results suggest that AIM and likely other domains in full-length VWF are in direct contact with the A1 domain and regulate the hemostatic properties of VWF.
Figure 1: Difference in HDX between isolated VWF A1 domains and full length VWF.
1 Deng W, Wang Y, Druzak SA, Healey JF, Syed AK, Lollar P, Li R. A discontinuous autoinhibitory module masks the A1 domain of von Willebrand factor. Journal of thrombosis and haemostasis : JTH . 2017. 10.1111/jth.13775.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal